“Mommm! I Hate Broccoli!”: Dietary Preferences and the Development of Taste
“Mommm! I Hate Broccoli!”: Dietary Preferences and the Development of Taste
Katelyn Chen Thomas Jefferson High School for Science and Technology
This article placed 5th in the 2022 Teknos Summer Writing Contest.
You’re a five-year-old, belly rumbling and ready for dinner… but your spirits fall at the sight of a menacing mound of broccoli on your plate. Why??!!! You cry out internally, but it’s no use. Your parents tell you to eat your greens — or else. Despite your protests and objections, you eventually force down bites of the gross-tasting vegetable. It’s a classic tale of childhood.
Yet every human being must supply their body with nutrients and vitamins, including the essential minerals, fiber, and disease-preventing chemicals found in cruciferous vegetables like broccoli [7]. So, why don’t we all enjoy leafy greens if they have so many health benefits?
Dietary preferences vary widely from person to person and can seriously affect long-term health; if you dislike certain vegetables to the point of avoidance, you could suffer from a lack of vitamins found in those vegetables. The impact of taste on diet can also be found in patients with dietary health conditions. Those with diabetes generally have lower taste sensitivity and may consume sweeter foods due to stunted taste [2]. Obesity is correlated with impaired sensitivity to fatty acid, which can influence overconsumption of fatty foods [10]. Dietary preferences can be affected by a range of factors from personal experiences to genetics. To understand the factors influencing taste, and whether you can happily devour a meal of Brussels sprouts and kale, we must start with how we taste.
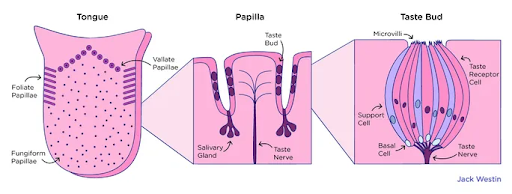
Your tongue is covered in papillae, which are projections visible as tiny bumps on the surface. Most contain taste buds (such as the “mushroom-like” fungiform papillae) but some do not and instead control tactile sensations. Generally, tasting papillae are found on the tongue and the soft palate — where you might feel “aftertaste” near the back of the roof of the mouth [9].
Figure 1. The types of papilla and a taste bud.
Each papilla contains up to 22 bulb-shaped taste buds of 50-150 taste cells [5]. These cells have fingerlike projections called microvilli which extend out of the taste bud through the taste pore. Certain chemicals in food, aptly called tastants, dissolve in your saliva and interact with microvilli to create the sensation of taste [9].
Researchers have confirmed five primary tastants: sweet, sour, bitter, salty, and umami (savory, or “deliciousness,” in Japanese), which comprise a wide variety of chemical compounds [1]. Their contact with taste buds triggers a signaling pathway that sends electrochemical impulses to the brain [9]. In the past, “labeled-line” theory was used to explain the neural response to taste: gustatory neurons in the brain would be specialized to respond to each tastant; for example, one would respond to “salty” signals and another to “sweet” signals. Research has since found that “across-neuron activity” theory is more accurate, in which neurons all contribute to producing information about multiple qualities of taste. Not only do individual neurons respond to multiple taste signals, but they also record the intensity and pleasurability of the taste. Thus, the brain interprets different tastes by generating unique activity patterns across a large set of neurons [6].
Now, let’s get back to our original question: why are leafy greens so loathed?
Genetics could be to blame. A single nucleotide polymorphism (SNP) — a change to a single nucleotide in the sequence for a gene in the bitter taste receptor gene TAS2R38 can cause individuals to perceive extreme bitterness in foods like cruciferous vegetables that contain chemicals from a group of compounds called thiourea. According to Bartoshuk et al. 1992, a study examining the perception of bitter tastes, about 25% of the population are “supertasters” with heightened taste abilities towards bitter foods, including cruciferous vegetables, as well as chili peppers and sugar. The rest of the population is made up of medium tasters and non-tasters, who do not taste certain bitter chemicals at all. The variability of TAS2R38 is strongly associated with taster status and can explain up to 85% of variation in taste perception; however, the effects of other genes and external factors have not been adequately explored.
Research on the influence of taster status on vegetable preference has seen varied results [5]. One study on college students found that tasters consumed more vegetables than non-tasters [3]. However, other studies showed no difference in vegetable intake across status, or that external factors like age and gender appeared to modify this relationship. Additionally, preference is ultimately a personal taste. Take dark chocolate as an example. You might find its bitter taste to be unpleasant, while another may enjoy it, despite your perceptions of the taste being similar [5].
It is also important to note the distinction between taste and flavor, which are often conflated. What people consider to be “taste” is usually “flavor”, the combination of signals from scent, mouthfeel (tactile sensation), and taste. On the other hand, taste is only what arises from taste cell signaling [9]. This article focuses on taste, but in some studies, it is near impossible to tease out the independent effects of the gustatory system (taste) sans any contributions from the olfactory (smell) or trigeminal (texture and temperature) systems. This complexity means it is difficult to make solid conclusions about the effects of taste [8]. Accordingly, further investigation into the entanglement of the various signals from different systems is key to understanding taste.
Your preferences could even be a consequence of how your mother ate during pregnancy. Taste bud patterns form during embryogenesis and the first functional taste cells develop during gestation. Throughout pregnancy, tastants from the maternal diet reach the amniotic fluid, which the fetus continuously swallows. After birth, tastants are also found in breast milk. This early exposure to the mother’s preferences heavily influences the dietary choices of the children, since the “feeding mode”, or dietary pattern, evolves rapidly during the first few years of life. Even though taste cells are routinely replaced throughout adult life, a healthy individual’s sense of taste is remarkably stable [1].
Alternatively, you may originally have had no ill will towards broccoli, but a distressing experience changed everything. This situation is called taste-aversion learning: if eating a food causes an adverse reaction, we learn to avoid it. The same phenomenon can occur in illnesses that inadvertently pair a taste with an unpleasant experience. Cancer patients’ loss of appetite after chemotherapy is typically caused by learned aversion due to gastrointestinal stress from the treatment [9]. In Roux-en-Y gastric bypass (RYGB) surgery, a treatment for weight loss, patients often report diet changes or eating less afterwards in part because many foods cause gastrointestinal discomfort. For instance, eating fats and simple sugars after RYGB surgery often causes “dumping syndrome”, a group of symptoms including diarrhea, nausea, and lightheadedness, because food moves too quickly from the stomach [4][8].
Besides explaining your eating habits, learning about the development of taste can also help adjust diets for individuals with conditions that change their taste preferences, create tastier health substitutes for individuals with dietary restrictions, and develop treatments for taste disorders that are frequently overlooked in medicine. Although taste is one of our basic senses, there is still much left to understand about it. Further research into the factors affecting development and subsequent variability of taste will no doubt hold valuable information for us — even if just to get kids to eat more veggies.
References
[1] Barlow, L. A., & Klein, O. D. (2015). Developing and regenerating a sense of taste. Neural Crest and Placodes, 401–419. https://doi.org/10.1016/bs.ctdb.2014.11.012
[2] Catamo, E., Robino, A., Tinti, D., Dovc, K., Franceschi, R., Giangreco, M., Gasparini, P., Barbi, E., Cauvin, V., Rabbone, I., Battelino, T., & Tornese, G. (2022). Altered taste function in young individuals with type 1 diabetes. Frontiers in Nutrition, 8. https://doi.org/10.3389/fnut.2021.797920
[3] Duffy, V. B., Hayes, J. E., Davidson, A. C., Kidd, J. R., Kidd, K. K., Bartoshuk, L. M. (2010). Vegetable intake in college-aged adults is explained by oral sensory phenotypes and TAS2R38 genotype. Chemosensory Perception, 3, 137–148. https://doi.org/10.1007/s12078-010-9079-8
[4] Dumping syndrome. (n.d.). National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved August 2, 2022, from https://www.niddk.nih.gov/health-information/digestive-diseases/dumping-syndrome/
[5] Feeney, E. (2011). The impact of bitter perception and genotypic variation of TAS2R38 on food choice. Nutrition Bulletin, 36, 20-33. https://doi.org/10.1111/j.1467-3010.2010.01870.x
[6] Lemon, C. H., & Katz, D. B. (2007). The neural processing of taste. BMC Neuroscience, 8. https://doi.org/10.1186/1471-2202-8-S3-S5
[7] Manchali, S., Chidambara Murthy, K. N., & Patil, B. (2012). Crucial facts about health benefits of popular cruciferous vegetables. Journal of Functional Foods, 4(1), 94-106. https://doi.org/10.1016/j.jff.2011.08.004.
[8] Mathes, C. M., & Spector, A. C. (2012). Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: A direct-measures approach. Physiology & Behavior, 107(4), 476–483. https://doi.org/10.1016/j.physbeh.2012.02.013
[9] Smith, D. V., & Margolskee, R. F. (2001). Making sense of taste. Scientific American, 284(3), 32–39. http://www.jstor.org/stable/26059127
[10] Stewart, J. E., Feinle-Bisset, C., & Keast, R. S. J. (2011). Fatty acid detection during food consumption and digestion: Associations with ingestive behavior and obesity. Progress in Lipid Research, 50(3), 225-233. https://doi.org/10.1016/j.plipres.2011.02.002
[11] [The types of papilla and a taste bud]. (n.d.). Jack Westin: The Complete MCAT Course. https://jackwestin.com/resources/mcat-content/other-senses/taste