Nanomedicine: Paving the Path Towards Better Targeted Drug Delivery
Nanomedicine: Paving the Path Towards Better Targeted Drug Delivery
Kavya Kuttuva Thomas Jefferson High School for Science and Technology
This article placed 3rd in the 2022 Teknos Summer Writing Contest.
In 1959, American physicist and Nobel Prize laureate Richard Feynman posed an interesting question: “Why can’t we write the entire 24 volumes of the Encyclopedia Britannica on the head of a pin?” This idea seemed unbelievable in that day and age and nothing more than a mere vision. Now, this idea can be translated into reality with nanotechnology [3]— the manipulation of matter on an atomic scale or molecular scale.
Currently, nanotechnology is commonly used to develop products made of polymer composite materials such as sunscreen, fabrics, and food packaging. Other practical applications include the incorporation of nanotechnology in electronics, from flash memory chips in smartphones and thumb drives to UHD displays in television [2].
A growing interest in the medical applications of nanotechnology led to the emergence of the field of nanomedicine, which has the potential to bring significant advances in the diagnosis, treatment, and prevention of disease. Nanomedicine is vital to precision medicine, an approach that considers the genetic and environmental differences between individuals. One common application of precision medicine is blood donations. Before Karl Landsteiner discovered blood types in 1901, blood transfusions were either a hit or miss [7]. If the blood a patient received wasn’t compatible with their blood type, they could die from an adverse immune system response. However, precision medicine offered a solution — in 1907, it was found that matching donor and recipient blood types greatly reduced the risk of rejection.
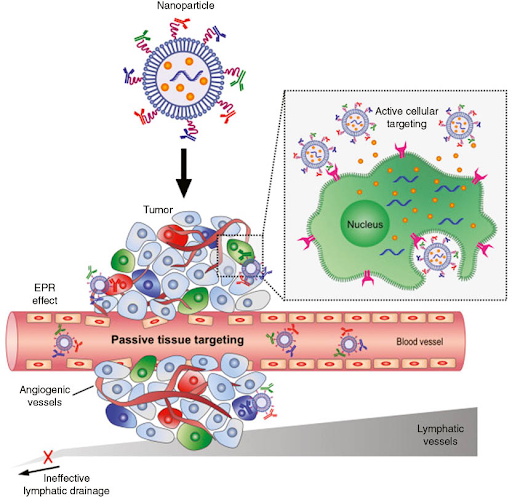
In today's world, nanomedicine can be applied to “targeted drug delivery,” a system in which drugs are selectively administered to a predetermined location (often cancer tissue). The efficacy of our current health treatments for cancer (radiotherapy and chemotherapy) is limited by the appearance of normal tissues adjacent to the tumor. These treatments come with side effects that weaken the patient's immune system [4]. Additionally, current cancer treatments have low specificity and limited targeting. [10]. Drug-carrying particles called nanocarriers address this issue. Nanocarriers can be modified to utilize passive and active targeting mechanisms to reach tumor tissues. Passive targeting incorporates the enhanced permeability and retention (EPR) effect, a natural phenomenon in which targeted drugs can progressively accumulate in tumor tissues [13]. High rates of proliferation in cancer cells lead to a weakened cell structure and weakened permselectivity in tissues, signifying that nanoparticles can be better retained in these systems. Permselectivity can be thought of as a pasta strainer; cells have systems to keep unwanted materials out, similar to how a pasta strainer strains out water. However, nanoparticles benefit from the low permselectivity of cancer cells, where the permeating nanocarriers are not removed efficiently and are localized in the cell microenvironment [9]. Inherently, there are limitations to nanoparticle-based passive targeting, as not every cancer cell universally experiences the EPR effect in the same way, and permeability varies depending on the tumor type.
Figure 1. Active and passive tissue targeting. Note how the passive tissue targeting system involves an ineffective lymphatic drainage system, which is a prominent hallmark of cancer cells [9].
The second type of nanoparticle targeting is active targeting. Active targeting is based on the interactions between receptors, proteins inside or on a cell’s surface, ligands, the molecules that bind to receptors. The interactions between receptors and ligands are analogous to those of a lock and key [6]. Scientists can alter the ligand (the key) attached to the nanocarriers based on the receptor (the lock) that they have to match with. There are a few common receptors on cancer cells, such as peptide-binding receptors, transferrin receptors (found in solid tumors), and folate receptors [12]. Nanomedicine is particularly helpful for active targeting, as scientists select the ligands to be incorporated into the nanocarrier. Once the ligand binds to the receptor (or the key fits into the lock), a cell process called receptor-mediated endocytosis is induced, enabling the release of the therapeutic drug in the cancer cell [12, 15].
Passive and active targeting are the two broad categories of targeted drug delivery — now what? What structures transport the nanoparticles that contain the drug to be released?
Currently, drug delivery involves a variety of non-nanotech-based systems, such as cell-penetrating peptides, a system in which short amino acids facilitate drugs to translocate across the plasma membrane [14]. Unfortunately, this system comes with concerns, such as whether delivery systems can be applied in vivo and as the fact that these cell-penetrating peptides may not bind selectively to disease-specific structures, potentially increasing drug exposure to healthy tissues [11]. Another commonly used mode of drug delivery is a class of vesicles called liposomes, however they are expensive to produce and have a shorter lifespan and stability. Liposomes are also likely to be targeted and cleared out by the body’s reticulo-endothelial system before they can make a real impact [14, 11].
A novel and promising method of nano-drug delivery is currently being tested at Harvard: DNA origami. When you think of origami, folded swans and flowers probably come to mind. While DNA origami isn’t quite an assortment of brightly colored animals, they do involve complex folds and organization. With DNA origami, a single strand or scaffold of a DNA-based nanoparticle can fold itself in various ways and bind to short DNA strands. These DNA nanoparticles are heavily customizable, accentuating their importance in precision-based medicine. When Harvard researchers tested the stability of engineered DNA nanoparticles in living cells, they found that the nanoparticles were efficiently absorbed and remained intact [5].
Using the DNA origami technique, we can design “nanobots” responsive to external stimuli and capable of containing single-stranded DNA or RNA ligands called aptamers that binds to a specific target [6]. Through these ligands, we can target cancer cells specifically. Once the nanobots recognize aptamers on cancer cells, the entire nanobot is transported into the cell via a process called receptor-mediated endocytosis. From there on, a series of cellular reactions ensure that the nanobot’s drug load can be released [1]. Similar to DNA origami is a nanotechnology known as DNA self-assembly. The main difference between the two systems is that an environmental cue is needed for a DNA structure to develop in DNA self-assembly. The drug would only be released once the DNA structure forms through nanosensors, still ensuring a precise delivery of drugs [1, 8].
Nanomedicine has shown immense potential to address challenges encountered in medical imaging, diagnosis, and treatment in several diseases. However, research into the the safety and tolerance of these new approaches is still imperative [15]. Further investigation must be conducted on the interactions between nanoparticles and our immune system, as the size, shape, and type of nanoparticle all affect the efficacy of the treatment. The development of a framework for the evaluation of nanomedicine is essential before it can be applied to patients and alter outcomes. Still, with the rise of nanotechnology, the future of the medicine is highly promising. What was once a dream is now closer to reality in improving the treatments of cancer, cardiovascular, and neurodegenerative diseases; from “nano” steps will truly emerge enormous changes.
References
[1] Abbas, A., & Wickham, S. (n.d.). DNA origami nanobots. The University of Sydney Nano Institute. Retrieved July 27, 2022, from https://www.sydney.edu.au/nano/our-research/research-programs/dna-origami-nanobots.html
[2] Applications of Nanotechnology. (2021, October 20). National Nanotechnology Initiative. Retrieved July 27, 2022, from https://www.nano.gov/about-nanotechnology/applications-nanotechnology
[3] Bayda, S., Adeel, M., Tuccinardi, T., Cordani, M., & Rizzolio, F. (2019). The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules, 25(1). https://doi.org/10.3390/molecules25010112
[4] Germain, M. G., Caputo, F., Metcalfe, S., Tosi, G., Spring, K., Åslund, A. K.O., Pottier, A., Schiffelers, R., Ceccaldi, A., & Schmid, R. (2020, July 15). Delivering the power of nanomedicine to patients today. Journal of Controlled Release, 326. https://doi.org/10.1016/j.jconrel.2020.07.007
[5] Hysolli, E. (2018, June 12). DNA origami suit up to thwart molecular villains. Wyss Institute. Retrieved July 27, 2022, from https://wyss.harvard.edu/news/dna-origami-suit-up-to-thwart-their-molecular-villains/
[6] Miller, E. J., & Lappin, S. L. (2022). Physiology, Cellular Receptor. In StatPearls. StatPearls Publishing.
[7] Nassar, S. F., Raddassi, K., Ubhi, B., Doktorski, J., & Abulaban, A. (2020). Precision Medicine: Steps along the Road to Combat Human Cancer. Cells, 9(9), 2056. https://doi.org/10.3390/cells9092056
[8] Patra, J. K., Das, G., Fraceto, L. F., Campos, E., Rodriguez-Torres, M., Acosta-Torres, L. S., Diaz-Torres, L. A., Grillo, R., Swamy, M. K., Sharma, S., Habtemariam, S., & Shin, H. S. (2018). Nano based drug delivery systems: recent developments and future prospects. Journal of nanobiotechnology, 16(1), 71. https://doi.org/10.1186/s12951-018-0392-8
[9] Seleci, M., Seleci, D. A., Joncyzk, R., Stahl, F., Blume, C., & Scheper, T. (2016). Smart multifunctional nanoparticles in nanomedicine. BioNanoMaterials, 17(1–2). https://doi.org/10.1515/bnm-2015-0030
[10] Sinha, R., Kim, G., Nie, S., & Shin, D. (2006). Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Molecular Cancer Therapeutics, 5(8). https://doi.org/10.1158/1535-7163.MCT-06-0141
[11] Skotland T, Iversen TG, Torgersen ML, Sandvig K. Cell-Penetrating Peptides: Possibilities and Challenges for Drug Delivery in Vitro and in Vivo. Molecules. 2015; 20(7):13313-13323. https://doi.org/10.3390/molecules200713313
[12] Worm, D. J., Els-Heindl, S., & Beck-Sickinger, A. G. (2020). Targeting of peptide-binding receptors on cancer cells with peptide-drug conjugates. Peptide Science, 112(3). https://doi.org/10.1002/pep2.24171
[13] Wu J. (2021). The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. Journal of personalized medicine, 11(8), 771. https://doi.org/10.3390/jpm11080771
[14] Xie, J., Bi, Y., Zhang, H., Dong, S., Teng, L., Lee, R. J., & Yang, Z. (2020). Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2020.00697
[15] Yao, Y., Zhou, Y., Liu, L., Xu, Y., Chen, Q., Wang, Y., Wu, S., Deng, Y., Zhang, J., & Shao, A. (2020). Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Frontiers in Molecular Biosciences. https://doi.org/10.3389/fmolb.2020.00193